Bespoke Glycosylation Signatures
NeuImmune is an early-stage biotechnology company founded in 2021 with leading scientists from the University of Maryland (UMD), University of California, San Diego (UCSD), and the Technical University of Denmark (DTU). Our focus is on developing innovative biopharmaceuticals and vaccines through the precision glycoengineering and delivery of biologics that address important medical and public health needs.
Contact us today for more information.
Slide title
Write your caption hereButton
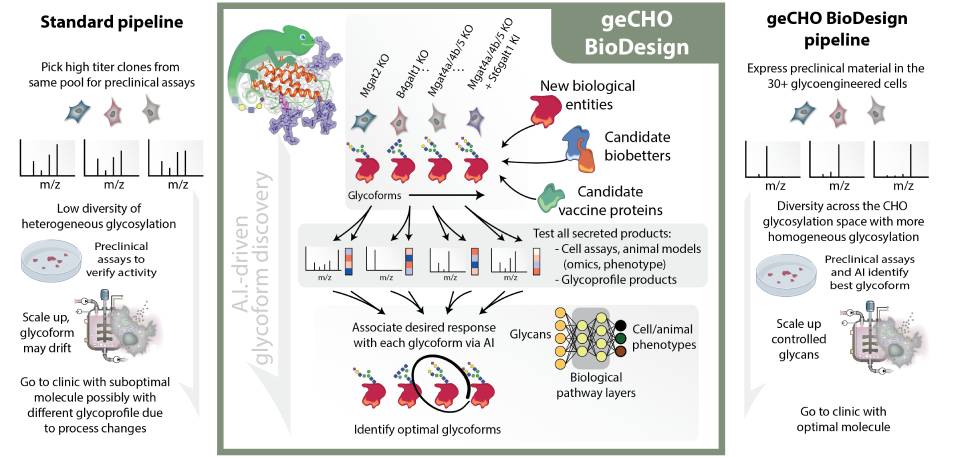
geCHO GlycoDesign Cell Technology
International sales of recombinant glycoproteins currently exceed $200B each year. The post-translational modifications on these therapeutic proteins can have extensive impacts on biologic efficacy, activity, stability, formulation, half-life, safety, and economics. In particular, protein glycosylation affects the function, safety, and half-life of diverse biologics.
- Read More
The development of this technology began with the sequencing of CHO-K1 cells and the hamster all CHO cells were derived from in 1957. This provided the basis to develop computational models of CHO cell metabolism, protein secretion, and glycosylation and allowed us to engineer complex traits into the cells, including a panel of GMP-ready glycoengineered CHO cells (geCHO). These cells, coupled with innovative artificial intelligence algorithms, form NeuImmune's Glyco-engineered CHO cell platform, enabling the discovery and production of optimal glycoforms in engineered CHO cell lines.
A significant challenge experienced by the pharmaceutical industry is designing and controlling therapeutic protein glycosylation. The glycans on a protein can significantly change its activity, stability, and serum half-life, and it is rarely clear which glycoform is optimal. Furthermore, heterologous recombinant expression of glycoproteins rarely results in a glycoform that mimics the glycosylation that has been selected through evolution in the natural tissue, as shown below when comparing natural glycoforms of liver secreted proteins and the glycoforms obtained when the proteins are expressed in CHO cells.
Searching for the optimal glycosylation, or even matching more natural glycosylation, can be difficult since few tools exist to allow one to adequately search the space of glycoforms to find optimal glycosylation for a drug of interest, despite data existing for dozens of proteins showing small changes in glycosylation can significantly affect drug safety and efficacy. For example, removing one fucose commonly found on glycans on human IgG1 provided more than a 50-fold increase in binding efficacy and antibody-dependent cellular cytotoxicity, thus substantially increasing drug potency of blockbuster monoclonal antibody drugs. Similarly, the increase of sialic acid on erythropoietin increased serum half-life from minutes to hours. These are just two examples that emphasize the importance of controlled glycosylation. Currently, there are dozens of mammalian proteins wherein the glycosylation has been carefully studied and found to impact protein function and half-life substantially.
NeuImmune is leveraging this platform to develop optimal novel drugs and biobetters, where the optimal glycoprofiles are unknown. To help discover these optimal glycoforms for New Molecular Entities (NMEs) and BioBetters, our platform presents a rapid means to develop diverse glycoprofile libraries. It deploys A.I. techniques to connect specific glycoprofile features to drug safety, efficacy, and manufacturing traits, thus guiding drug discovery and development. If optimal glycoforms are found, such as the 50X potency of IgGs or the increased half-life of erythropoietin described above, are found, the dosing and frequency of a novel treatment can be drastically reduced thus requiring substantially less material to treat each person. Given that manufacturing costs account for roughly 15-20% of a therapeutic's total cost, this could lead to hundreds of millions of dollars in savings to the company each year. The new drug or biobetter is on the market.
In addition to enhancing new drug efficacy, this platform will be of substantial economic value for new drugs and biobetters. Specifically, our cell lines provide more homogeneous glycoforms that are constrained by genetic modifications.
Furthermore, our algorithms and technologies provide a means for tight control of a product's glycoprofile both from the engineered cell lines and algorithm-guided control of glycans using metabolite feeds. These technologies have the potential of extending the franchise of new drugs by making it increasingly difficult to match a glycoprofile without using the same genetic basis for a cell line and algorithmic control found in our pipeline products or partnered products. This franchise extension can thus maintain $100M-$1B revenues each month for a blockbuster innovator drug, NeuImmune's geCHO GlycoDesign technology platform presents an AI-infused platform for glycoengineering to accelerate drug discovery and guide host cell design for manufacturing.
Our geCHO GlycoDesign Cells
- NeuImmune’s geCHO GlycoDesign: a platform for finding optimal glycoforms
For most biologics, optimal glycoprofiles are unknown, so glycosylation is often not considered in the drug development pipeline. Thus, highly potent and safer forms remain unidentified. Using the NeuImmune Glyco-engineered CHO cell technology can create a cell panel of 30 glycoengineered CHO cells; NeuImmune produces different therapeutic glycoprotein variants with defined, homogenous, and predictable glycoforms. These proteins can be made within the same timeframe of the most rapid commercial pipelines. Still, the dozens of variants comprehensively span the glycan design space available with CHO cells instead of just representing a few heterogeneous glycoforms obtained using standard approaches. The produced glycoprotein variants can be tested in relevant assays for activity, stability, and half-life to identify the best possible variant.
Our A.I. can be further used to integrate glycan profiles of the drug panel with assay results to identify which glycans underlie the desired therapeutic response. Our Markov modeling methods can predict further cell modifications needed to enhance desired drug attributes.
- The technology behind the geCHO GlycoDesign cell platform
NeuImmune’s Glyco-engineered CHO cell platform has two major components: the geCHO cell line panel and the A.I. for identifying optimal glycoforms and cell line optimization.
The geCHO cell line panel: Typical drug development procedures for new molecular entities often express the candidate proteins in a standard platform cell line and generally only tests the glycoprofiles that come out of the cell line of interest. Thus, state-of-the-art pipelines do not obtain nor test alternative glycoforms. However, studies have shown for 100s of proteins that changes in their glycosylation can considerably affect the efficacy, half-life, or safety of secreted proteins and biotherapeutics. Our geCHO panel can produce diverse glycoforms that span the diversity of glycans obtainable in CHO cells.
Our geCHO panels are built from a GMP-ready CHO-S parental line that has been adapted to serum-free suspension growth. This panel has been genome-edited deeply phenotyped with omics data and other phenotypic measures. In each cell line, combinations of 17 different genes have been deleted or knocked in to make more homogeneous glycoprofiles, including most N-glycan structures attainable in CHO cells. This includes N-glycans that are high mannose, monoantennary, bi-antennary, tri-antennary, tetra-antennary, fucosylated, afucosylated, a-2,3-sialylated, a-2,6-sialylated, asialylated, etc. Thus, our panel of geCHO mutants produces a comprehensive range of different glycoforms attainable in CHO cells.
- A.I. for identifying glycans linked to optimal drug performance:
Once the geCHO cells are used to generate a panel of different glycoforms, which are used for preclinical tests, the different glycoforms and preclinical metrics can be analyzed together to identify the glycans and glycan features associated with optimal drug performance. The assumptions underlying standard machine learning approaches can be invalid when used for analyzing glycosylation. Specifically, the algorithms depend on assumptions that each glycan is independent and unconnected. However, all glycans are co-dependent and require one to account for all associations between glycans to interpret machine learning models accurately and maximize statistical power. Thus, our pipeline's core includes a unique explanatory A.I. system that leverages a unique mix of machine learning algorithms with a powerful systems biology framework to identify the specific glycans, glycan substructures, and epitopes that are provide the desired response in glycoprotein activity and half-life.
- A.I. for refining host cell glycosylation for optimal drug performance:
Once optimal glycoforms are identified, the complexity of glycan biosynthesis in mammalian cells makes it often extraordinarily difficult to know how cells could be perturbed to increase the optimal glycoform homogeneity. To achieve this, we have developed a systems biology framework that leverages probabilistic Markov modeling and optimization algorithms to identify which enzymes to target to enhance a desired host cell lines.

Slide title
Write your caption hereButton

Slide title
Write your caption hereButton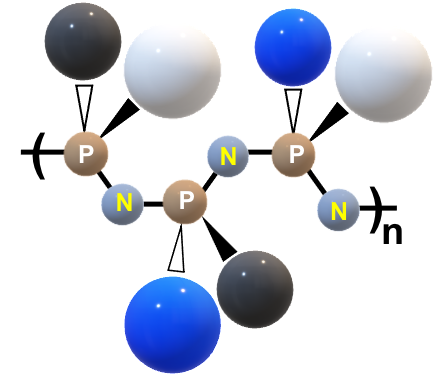
Slide title
Write your caption hereButton
Polyphosphazene Technology – Delivery
NeuImmune has developed a novel approach to developing immunoadjuvants and drug delivery carriers utilizing the power of synthetic polyphosphazene (PPZ) chemistry. Polyphosphazenes, a large class of macromolecules based on the phosphorus-nitrogen backbone and organic side groups, provide an ideal foundation for synthesizing biodegradable polymers with a sophisticated set of design parameters. They possess a unique combination of features distinguishing them from other classes of biomedical polymers – high functional density, extraordinary flexibility of the backbone, tunable biodegradation, and unprecedented structural versatility stemming from their unusual synthetic route.
- Read More
The resulting multifunctionality, ability to spontaneously self-assemble with proteins and biological systems, mimicry of biological materials, and built-in environmental sensitivity provide ample opportunities for the design of smart delivery carriers. Moreover, the novel synthetic methodologies allow both the high throughput discovery of new materials and manufacturing-friendly processes.
This approach already engendered the development of potent vaccine carriers based on water-soluble ionic polyphosphazenes. They have been proven to display a dramatic immunopotentiating effect in vivo manifested in improved magnitude, quality, onset, and duration of immune responses, in addition to the underlying vaccine sparing effect. Polyphosphazene adjuvants' unique position stems from an exceptional antigen-binding feature of these water-soluble macromolecules, which mimic dimensions of viruses and display multiple copies of the associated antigen, as well as "danger signals" needed for effective immune stimulation. Such multivalent presentation, in which antigens and receptor agonists are presented to the immune system in a repetitive array, has been shown to increase immune responses' potency. The lead polyphosphazene adjuvant – poly[di(carboxylatophenoxy)phosphazene], PCPP has been advanced into clinical trials and reported to be safe and immunogenic in humans. New generation of polyphosphazene adjuvants was generated, including potent structural derivatives and those with non-covalently attached Toll-Like Receptor (TLR) agonists. The technology has been further advanced to include important delivery modalities, such as functionalized nanoparticulate assemblies and polyphosphazene microneedles for transdermal delivery of biologics.
Polyphosphazene Technology – Stability
NeuImmune is also working with a new class of macromolecular derivatives, called PEGylated polyphosphazenes, offer a pioneering alternative to PEGylation that eliminates some of the undesirable effects of chemical conjugation and preserves the function of the therapeutic protein. PEGylation – a technology based on polyethylene glycol (PEG) attachment to the protein surface, is one of the most successful techniques addressing some of these shortcomings. PEGylation has been critical to prolonging the time these therapeutic proteins circulate in the blood and has achieved significant commercial success.
- Read More
"Non-covalent PEGylation" is a faster and easier approach to modifying therapeutic proteins and has several advantages over current approaches, including (a) an innovative "mix-and-use" bioproduction approach that can be included in existing manufacturing processes, (b) a broader scope of protein drugs to which this technology can be applied, (c) contaminant-free formulations, (d) prolonged time in circulation, and (e) dramatic reduction in manufacturing labor, equipment, and cost. Preliminary studies of proteins modified by PEGylated polyphosphazenes have shown improved protein stability, reduced undesirable immune responses, and better uptake by cancer cells.
The product pipeline for polyphosphazene macromolecules includes immunoadjuvants for HCV, SARS-CoV-2, and RSV vaccines. PEGylated polyphosphazenes will be developed in the field of non-covalent protein stabilization and therapeutic protein delivery.

Slide title
Write your caption hereButton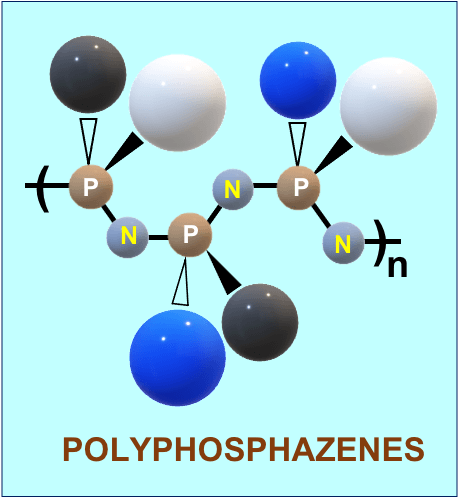
Slide title
Write your caption hereButton
